For the past nine months, researchers have been racing to create a vaccine to effectively prevent COVID-19. Various government health agencies have come together to work towards a goal of safely distributing 300 million doses of a COVID-19 vaccine by January 2021. This effort is known as Operation Warp Speed (OWS). OWS has provided resources to fast track the development of COVID-19 vaccines.
Even with OWS in place, creating an effective vaccine takes an immensely large amount of time and resources. Viruses are not really living organisms while bacteria are. This is the main reason why only a limited amount of treatments is available for viruses compared with the abundance of antibiotics to treat bacterial infections.
Viruses need a host, such as the human body, to reproduce. Therefore, treating a virus with a drug could potentially harm a human’s body. Zachary A. Klase, associate professor of biology at the University of the Sciences, told the Philadelphia Inquirer, “You want something that targets the sickness and not you. You need to look for the special things that only the virus is doing.”
What is an RNA Vaccine?
The vaccine candidates for COVID-19 are RNA vaccines, which comprise part of the virus’s genetic code in lipid particles. It is then introduced into a human’s body to tell cells what to build in order to protect from the virus. The following graphic explains how an RNA vaccine works.
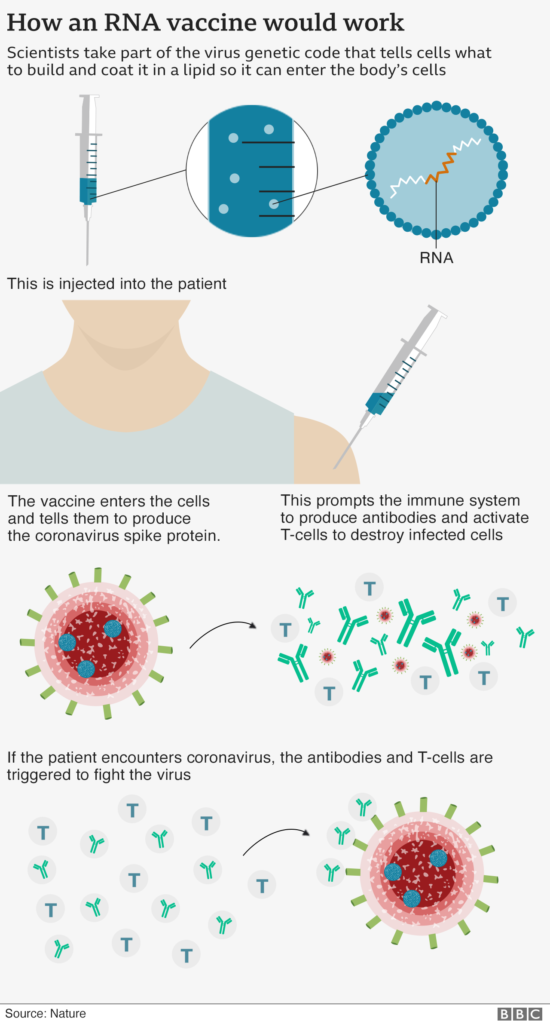
Who is Working on COVID-19 Vaccines?
Pfizer and BioNTech duo announced on November 9, 2020, that its vaccine candidate, BNT162b2, has 90% efficacy in preventing COVID-19. A week later, Moderna announced its vaccine candidate, mRNA-1273, to be 94.5% effective. Pfizer and BioNTech have since concluded their phase 3 study with results showing the vaccine candidate to be 95% effective. Specifically, the candidate has presented to be 94% effective in preventing COVID-19 in adults over the age of 65 years. Although these results are encouraging, a vaccine will not be ready for distribution until further testing and analyses of safety are complete.
There are several other organizations throughout the world who are working on vaccines as well. 54 vaccines are in clinical trials on humans, and about 87 are under active investigation on animals, according to the NYTimes COVID-19 Tracker.
What are the Main Concerns with COVID-19 Vaccines?
Distribution of the vaccine is a big concern for health care professionals. Sub-zero temperatures are necessary to store the vaccine. Certain areas of the country may have challenges keeping this temperature under control. For example, areas with a consistently high heat index or unreliable electricity may need additional resources for the distribution of the vaccines. The Pfizer vaccine can become useless if its temperature rises above -70 degrees Celsius. Moderna’s vaccine may be a better option for those areas due to its required storage temperature of -20 degrees Celsius.
Hospitals and health centers have already had to adjust their spending when COVID-19 first impacted them. The lack of funding provided to purchase the coolers and industrial freezers needed for transporting and storing the vaccine worries health care professionals.
The Center for Disease Control (CDC) has published an updated Interim Playbook for Jurisdiction Operations on October 29, 2020, and stated that an addendum including specific storage, handling, and transport information will be added.
Even if the vaccine distribution concerns are put at ease, there is still a question of whether enough people are willing to try out the vaccine to establish herd immunity. Herd immunity happens when enough people within a community are immune to a virus, making it harder for the virus to spread from person to person. SSRS conducted a survey of 1,205 people from October 1, 2020, to October 4, 2020. Results show that 51% of respondents would try to get vaccinated once it is approved. 45% said they would not try.
What Health Professionals Are Saying
“This is better than Christmas. The interim results of these two exciting mRNA vaccine candidates over the past week are encouraging, hopeful, and move us massively forward to getting out of this pandemic.”
Dr. Monica Gandhi, professor of medicine at the University of California – San Francisco
At a webinar hosted by Chatham House on Thursday, Dr. Anthony Fauci stated that “help is on the way, but it isn’t here yet. I doubt we are going to eradicate this. I think we need to plan that this is something we may need to maintain control over chronically. It may be something that becomes endemic, that we have to just be careful about.”
“Certainly, it is not going to be a pandemic for a lot longer because I believe the vaccines are going to turn that around.”
Dr. Anthony Fauci, Director of National Institute of Allergy and Infectious Diseases